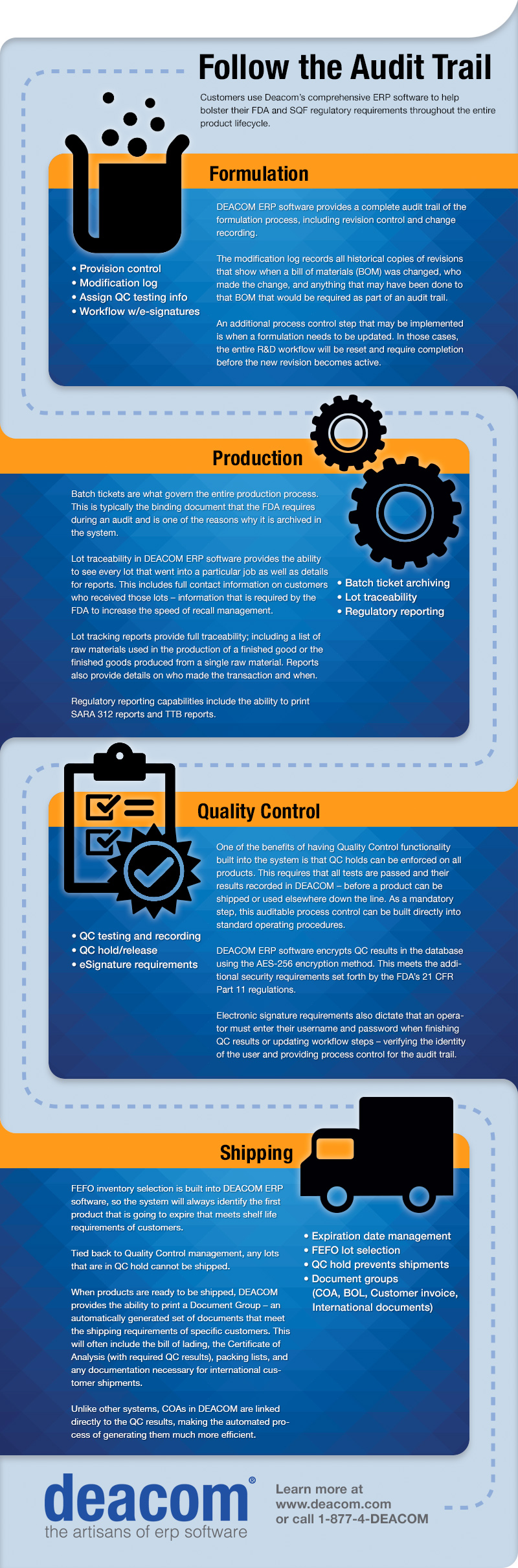
Strict adherence to the Food and Drug Administration’s (FDA) and Safe Quality Food Institute’s (SQF) regulatory requirements, and the ability to report on them when necessary, is of great importance to many of our customers who do business in industries regulated by these governing bodies. As providers of an ERP solution with the largest functional foundation, Deacom has built into its software the process controls and audit trail capabilities necessary for our customers to meet these requirements.
We’ve put this infographic together to illustrate exactly how Deacom’s ERP software accomplishes this throughout the entire product lifecycle.