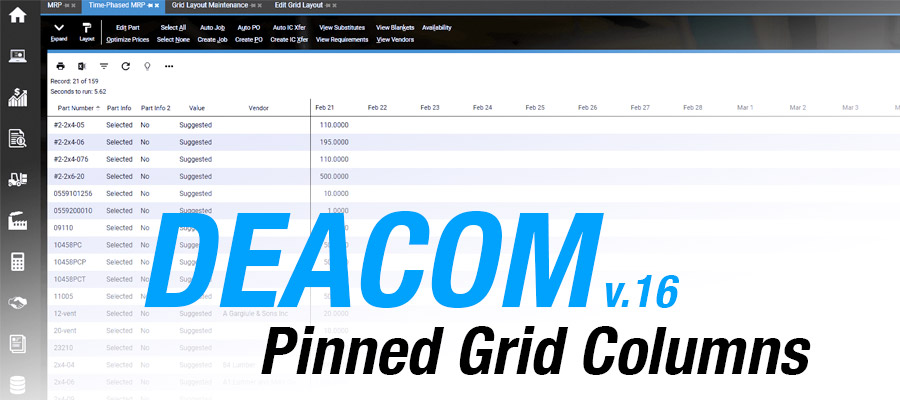
Deacom has recently added a new feature that enables grid columns to be pinned to the left side of a management report. Using the DEACOM grid layout tool, users can configure where the grid columns should be pinned in a management report by default. The pinned location can then be slid around once the management …
Continue reading “Pinned Grid Columns: Small Feature, Big Win”